Class 12 chemistry practice paper 2023
To increase more accuracy in CBSE Board exam 2023 you should definitely try to mock test of Class 12 chemistry practice paper 2023.
SEC-A
(SECTION A consists of 16 multiple choice questions carrying 1 mark each.)
1-The formation of cyanohydrin from a ketone is an example of
(a) electrophilic addition
(b) nucleophilic addition
(c) nucleophilic substitution
(d) electrophilic substitution
2-Which of the following is the IUPAC name of the chemical in which an ethyl group replaces one hydrogen of ammonia?
a) Ethanamine
b) Aminoethane
c) Ethylamine
d) Ethane amine
3-Which of the following statements about starch is incorrect?
a) Its soluble in warm water
b) It is a polymer of α-D-glucose
c) It is a reducing carbohydrate
d) It consists of branched chains
4-Which of the following statements about a lead storage cell (or a lead-acid battery) is false?
a) It is a primary cell
b) The cathode is made up of lead(IV) oxide
c) The anode is made up of lead
d) The electrolyte used is an aqueous solution of sulphuric acid
5-Which reagents are required for one step conversion of chlorobenzene to toluene?
(a) CH3Cl / AlCl3
(b) CH3Cl, Na, Dry ether
(c)CH3Cl/Fe dark
(d) NaNO2/ HCl /0-50oC
6-Which of the following has magnetic moment value of 5.9?
(a) Fe2+
(b) Fe3+
(c) Ni2+
(d) Cu2+
7-Consider the given figure and mark the correct option.
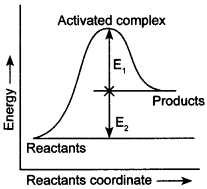
(а) Activation energy of forward read ion is E1 + E2 and product is less stable than reactant.
(b) Activation energy of forward reaction is E1 + E2 and product is more stable than reactant.
(c) Activation energy of both forward and backward reaction is E1 + E2 and reactant is more stable than product.
(d) Activation energy of backward reaction is E1 and product is more stable than reactant.
8-Acetone combines with ethylene glycol in dry HCl gas to generate
a) hemiacetals
b) cyclic ketals
c) cyclic acetals
d) acetals
9-The reaction of ethyl formate with an excess of CH3MgI followed by hydrolysis gives
(a) ethanol
(b) n-propyl alcohol
(c) propanal
(d) isopropyl alcohol
10-The activation energy of a reaction can be determined from the slope of which of the following graph:
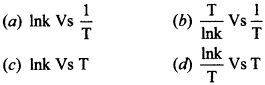
11-Phenol can be distinguish from ethanol by the reaction with ___
a)Br2/Water
b)Na
c)Glycerol
d)All of the Above
12-Although Zirconium belongs 4d transition series and hofnium to 5d transition series even they show similar physical and chemical properties because
a)Both belongs to the d- block
b)Both have same number of electrons
c)Both have similar atomic radius due to lanthanide contraction.
d)Both belong to the same group of the periodic table
Select the most appropriate answer from the options given below:
Both A and R are true and R is the correct explanation of A
Both A and R are true but R is not the correct explanation of A.
A is true but R is false.
A is false but R is true.
13-Assertion : In case of phenol, bromination takes place even in absence of Lewis acid whereas bromination of benzene takes place in presence of Lewis acid like FeBr3.
Reason : – OH group attached to benzene ring is highly deactivating.
14-Assertion (A): Benzoic acid doesn’t undergo Friedel-craft’s reaction.
Reason (R): Benzoic acid is an activating group and undergo electrophilic substitution reaction.
15-Assertion (A): Sucrose is called an invert sugar.
Reason: On hydrolysis, sucrose bring the change in the sign of rotation from dextro (+) to leavo(–)
16-Assertion: On increasing dilution, the specific conductance keep on increasing.
Reason: On increasing dilution, degree of ionisation of weak electrolyte increases and molality of ions also increases.
CBSE Chemistry Sample paper 2023 for Practice
SECTION – B
(SECTION B consists of 5 very short answer questions carrying 2 marks each)
17-The rate constant of a reaction A → B is 0.6 × 103 mol S-1 . If the concentration of [A] is 5 M, then what will be concentration of [B] after 20 months?
18-An aromatic compound “A” on heated with NaNO2+ HCl produces Benzene diazomium chloride, which on further treatment with Cu2Cl2 produces an molecule “B”. While B undergoes coupling reaction to produce biphenyl with an reagent C.
Mention A ,B & C and mention name of the coupling reaction occurred between B & C
19-a) Define Azeotrope with an example.
b) Mention the type of deviation from ideal behavior shown by solution of phenol and aniline and why?
19-An aromatic compound “A” on heated with NaNO2+ HCl produces Benzene diazomium chloride, which on further treatment with Cu2Cl2 produces an molecule “B”. While B undergoes coupling reaction to produce biphenyl with an reagent C.
Mention A ,B & C and mention name of the coupling reaction occurred between B & C
20-
Aldehyde, Ketone and Carboxylic acids
Write the reactions involved in the following reactions:
(i) Clemmensen reduction
(ii) HVZ reaction
OR
Do the following conversions in not more than two steps:
(i) Benzoic acid to benzaldehyde
(ii)Propanone to Propene
21-
i)Write the structure of the product obtained when glucose is oxidised with nitric acid.
ii)What are the products of hydrolysis of sucrose?
iii) Which component of starch is a branched polymer of a-glucose and insoluble in water?
Class 12 chemistry Mock test 2023
SEC – C
(SECTION C consists of 7 short answer questions carrying 3 marks each)
22-
(a) [CrCl2(en)2]Cl? Write the IUPAC Name.
(b)On the basis of CFT,write the electronic configuration of d5 ion if Δ0<P.
(c)[Cr(NH3)6]3+ is paramagnetic but [Ni(CN)4]2- is diamagnetic. Explain why?
23-A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential is measured 0,422 V. Determine the concentration of silver ion in the cell.
Given : E°Ag+/Ag = + 0.80 V, E° Cu2+/Cu = + 0.34 V. (Antilog 1.14493=13.93)
24-a) How do you convert Propan-2-ol to 2-methylpropan-2-ol
b) State Reimer-Tiemann reaction
c) Phenol is more acidic than ethanol, explain.
25-
An alkene ‘A’ (Mol. formula C5H10) on ozonolysis gives a mixture of two compounds, ‘B’ and ‘C’. Compound B’ gives positive Fehling’s test and forms iodoform on treatment with I2 and NaOH. Compound C’ does not give Fehling’s test but forms iodoform. Identify the compounds A, B, and C. Write the reaction for ozonolysis and formation of iodoform from B and C.
OR
a)Write IUPAC name of the following :
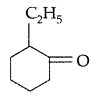
b) Write the equations involved in Wolff-Kishner reduction.
(c) What is formed when Tolune undergo treatment with chromyl chloride followed by hydrolysis?
26-Answer the following questions:
(a) What is essential and nonessential amino acids?
Write one example of each.
(b) What is the difference between a nucleoside and a nucleotide?
27-a) Chloroform is stored in dark coloured bottles.
b)Mention the product formed.
Class 12 chemistry practice paper 2023
28-a) The rate of a reaction becomes four times when the temperature changes from 293 K to 313 K. Calculate the energy of activation (Ea) of the reaction assuming that it does not change with temperature. [R = 8.314 JK-1mol-1, log 4 = 0.6021]
b) Define Pseudo first order reaction with examples.
Before proceeding to Case based Questions, you can checkout Class 12 SOLUTION chapter Important QnA
SEC-D
(SECTION D consists of 2 case-based questions carrying 4 marks each)
29-
Werner’s theory of complex compounds says every metal atom or ion has primary valency (oxidation state) which is satisfied by -vely charged ions, ionisable where secondary valency (coordination number) is non-ionisable, satisfied by Iigands (+ve, -ve, neutral) but having lone pair. Primary valency is non-directional, secondary valency is directional. Complex compounds are name according to IUPAC system. Valence bond theory helps in determining shapes of complexes based on hybridisation, magnetic properties, outer or inner orbital complex.
Complex show ionisation, linkage, solvate and coordination isomerism also called structural isomerism. Some of them also show stereoisomerism i.e. geometrical and optical isomerism. Ambidentate ligand are essential to show linkage isomerism. Polydentate Ligands form more stable complexes then unidentate Iigands. There are called chelating agents. EDTA is used to treat lead poisoning, cis-platin as anticancer agents. Vitamin B12 is complex of cobalt. Hemoglobin, oxygen carrier is complex of Fe2+ and chlorophyll essential for photosynthesis is complex of Mg2+.
(a) One mole of CrCI3 . 6H2O reacts with excess of AgNO3 to yield 2 mole of AgCI. Determine the Molecular formula of complex, do the IUPAC name of the compound.
OR
(a) What is hybridization, shape and magnetic properties of [CoF6]3- [Co = 27].
(b) Out [Fe(CO)5], [Fe(C2 O4 )3]3-, [Fe(H2O6)3+, [Fe(CN)6]3-, which is most stable?
(c) Out Cis – [Pt(en)2 CI2 ]2+ and trans (Pt(en)2CI2 )2+ which one shows optical isomerism?
30-
Metallic conductance involves movement of electrons where as electrolytic conductance involves movement of ions. Specific conductance increases with increase in concentration where as Am (molar conductivity) decreases with increase in concentration. Electrochemical cell converts chemical energy of redox reaction into electricity. Mercury cell, Dry cells are primary cells where as Ni-Cd cell, lead storage battery are secondary cells.
Electroehemical series is arrangement of elements in increasing order of their reduction potential. Electrolytic cell converts electrical energy into chemical energy which is used in electrolysis. Amount of products formed are decided with the help of Faraday’s laws of Electrolysis. Kohlrausch law helps to determine limiting molar conductivity of weak electrolyte, their degree of ionisation (α) and their dissociation constants.
(a) Out of 0.5 M, 0.01 M, 0.1 M and 1.0 M which solution of KCl will have highest value of specific conductance?
(b) Write the product of electrolysis of aq. NaCI on cathode.
(c) For an electrochemical cell Mg(s) + 2Ag+(aq) → 2Ag(s) + Mg2+. Give the cell representation and write Nernst equation.
OR
(c) State Kohlrausch’s law with examples
To Build up the confidence Give mock test once of this Class 12 chemistry practice paper 2023
SEC-E
(SECTION E consists of 3 long answer questions carrying 5 marks each)
31-
Explain the following observations : (Any five)
(a) Generally there is an increase in density of elements from titanium (Z = 22) to copper (Z = 29) in the first series of transition elements.
(b) Transition elements and their compounds are generally found to be good catalysts in chemical reactions.
(c)The chemistry of actinoids is not so smooth as that of lanthanoids
(d) The ionization enthalpies (first and second) in the first series of the transition elements are found to vary irregularly.
(e) Represent the oxidising action of potassium dichromate and write the ionic equations for its reaction with iodine.
(f) Zinc is not regarded as a transition element.
(g)Transition elements generally form coloured compounds
32-a) Three molecules of a solute (A) associate in benzene to form species A3. Calculate the freezing point of 0.25 molal solution. The degree of association of solute A is found to be 0.8. The freezing point of benzene is 5∘C and its Kf value is 5.13 K/m.
b) Define mole fraction.
c)1 Molar and 1 molal solution heated simultaneously, which one’s value will remain unchanged after heating? Why?
OR
a) An aqueous solution containing 3.12 g of barium chloride in 250 g of water is found to be boil at 080C Calculate the degree of dissociation of barium chloride. Given molar mass BaCl2= 208gmol−1, Kb for water = 0.52 K/m
b) Define the term ‘Reverse osmosis’.
c) Between 1 molar aqueous solution and 1 molal aqueous solution, which contains more volume of Solvent? Explain
33-a) An aromatic compound ‘A’ on treatment with aqueous ammonia and heating forms compound ‘B’ which on heating with Br2and KOH forms a compound ‘C’ of molecular formula C6H7 Write the structures and IUPAC names of compounds A, B and C.
b) Write the structures A, B and C in the following
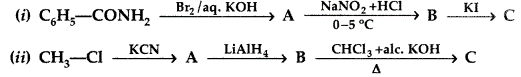
OR
a)Aniline does not give Friedel-Crafts reaction.
b) Distinguish chemically between Methylamine and Dimethylamine
c) Arrange the following in increasing order of pKb value:
C6H5NH2, C6H5NHCH3, C6H5N(CH3)2
d) Describe Carbylamine reaction
e) Draw the structure of Phenyl acetamide
——-X——-
All The best Folks
Link to download the above Class 12 chemistry practice paper 2023
Frequently Asked Questions(FAQs)
Chloroform is stored in dark coloured bottles.
Chloroform gets oxidsed slowly by air in the presence of light to an extremely poisonous gas phosgene. Therefore to avoid any exposure to air and sunlight, it is kept in dark coloured bottles.
Write the IUPAC Name of [CrCl2(en)2]Cl
Dichlorobis (ethane-1, 2-diamine) chromium (III) chloride
Between 1 molar aqueous solution and 1 molal aqueous solution, which contains more volume of Solvent? Explain
1 molal solution strength will remain unchanged as during heating mass of solvent don’t change.
What is the difference between a nucleoside and a nucleotide?
Nucleoside : A nucleoside contains only two basic components of nucleic acids i.e. a pentose sugar and a nitrogenous base. During their formation 1-position of the pyrimidine or 9-position of the purine moitey is linked to C1 of the sugar (ribose or deoxyribose) by a β-linkage.
On the basis of CFT,write the electronic configuration of d5 ion if Δ0<P.
Ans: t32g and e2g

Comments are closed.