CBSE Class 12 chemistry exam was conducted on 27th February 2024 and here you can find the original CBSE Class 12 chemistry question paper 2024.
This set question paper is of the 56/3 zone with all three sets of Chemistry Paper.
You can see questions and download the pdf of CBSE Class 12 chemistry Question paper 2024. All Three sets were prepared to conduct the smooth and fair examination practice. This year questions were Average to Easy type as per the student’s feedback.
First Start solving the 56/3/1 set-1 question paper
CBSE Class 12 chemistry question paper 2024: Set-1
1. Which of the following does not show variable oxidation states?
(A) Fe
(B) Cu
(C) Mn
(D) Sc
2. The type of isomerism shown by the complex [CoCl2(en)2] is
(A) Ionisation isomerism
(B) Geometrical isomerism
(C) Linkage isomerism
(D) Coordination isomerism
3. Which of the following is diamagnetic in nature?
(A) c o^ 3+ octahedral complex with strong Geld ligand
(B) c o^ 3+ octahedral complex with weak field ligand
(C) c o^ 3+ in a square planar complex
(D) C o^ 3+ , in a tetrahedral complex [Atomie number C_{0} = 271
4. Consider the following reaction:
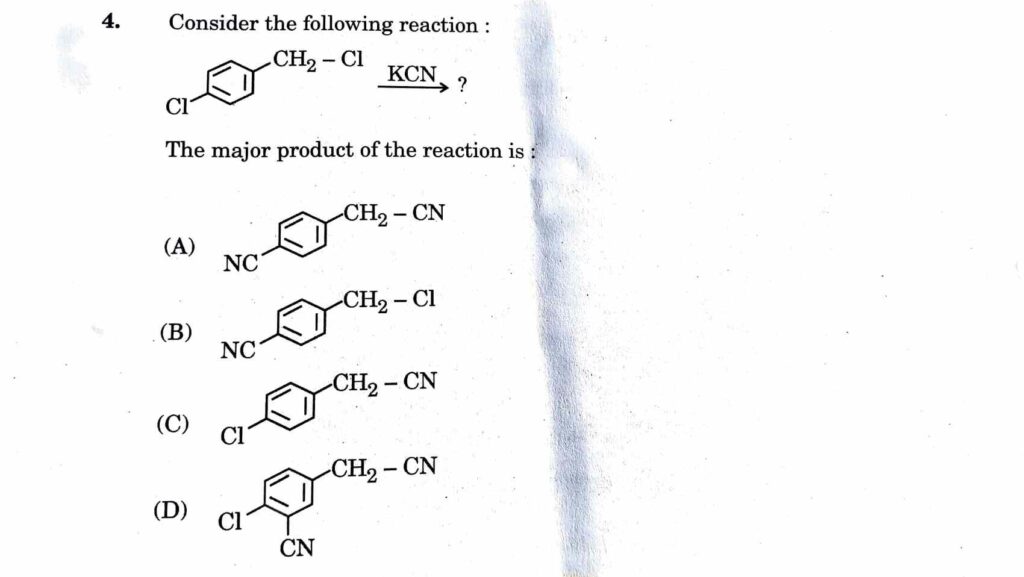
5. Which one of the following compounds has the lowest pK, value”
(A) p-Cresol
(B) p-Nitrophenol
(C) m-Nitrophenol
(D) 2,4,6-Trinitrophenol
6. (CH3)CH-O-CH3 , when treated with HI gives
(A) (CH3)2 CH-I + CH3OH
(B) (CH3)3 CH-OH + CH3-I
(C) (CH3)2CH-I+ CH3-I
(D) (CH3)2CH-OH+CH3OH
7. Which of the following compounds on treatment with benzene sulphonyl chloride forms an alkali-soluble precipitate?
(A) CH3-CO-NH2
(B) (CH3)3N
(C) (CH3)2NH
(D) CH3CH2NH2
8. The order of increasing basicities of CH3NH2 (I), (CH3)2NH (II), (CH3)3N (III), C6H5NH2 (IV)
A) IV < I < II
(B) II < I < III
(C) I<IIIII< IV
(D) II < III < I < IV
9. The vitamin which plays an important role in coagulating blood is:
(A) Vitamin A
(1) Vitamin E
(C) Vitamin D
(D) Vasmin K
10. When a catalyst increases the rate of a chemical reaction, then the rate constant (k)
(A) remains constant
(B) decreases
(C) incraeses
(D) may increase or decrease depending on the order of the reaction
11. A 1% solution of solute ‘X’ is isotonic with a 6% solution of sucrose (molar mass=342 g/mol. The molar mam of solute ‘X’ is:
(A) 34.2 g/mol
(B) 57 g/mol
(C) 114 g/mol
(D) 3-42 g/mol
12. During the electrolysis of aqueous NaCl, the cathodic reaction is
(A) Oxidation of Cl- ion
(B) Reduction of Na+ ion
(C) Oxidation of H2O
(D) Reduction of H2O
For Questions number 13 to 16, two statements are given-one labeled as Assertion (A) and the other labeled as Reason (R). Select the correct answer to these questions from the codes (A), (B), (C) and (D) as given below
(A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
(B) Both Assertion (A) and Reason (K) are true, but Reason (R) is not the correct explanation of Assertion (A).
(C) Assertion (A) is true, but Reason (R) is false.
(D) Assertion (A) is false, but Reason (FR) is true.
13. Assertion (A): Addition of ethylene glycol to water lowers its freezing point.
Reason (R): Ethylene glycol is insoluble in water due to the lack of its ability to form hydrogen bonds with water molecules.
14. Assertion (A): Order of reaction and molecularity are always same for complex reactions.
Reason (R): Order is determined experimentally and molecularity is applicable only for elementary reactions
15. Assertion (A): The boiling point of ethanol is higher than that of dimethyl ether,
Reason (R): Ethanol molecules are associated through hydrogen bonding whereas in dimethyl ether, it is not possible.
16. Assertion (A): Aniline does not undergo Friedel-Crafts reaction.
Reason (R): Friedel-Crafts reaction is an electrophilic substitution reaction.
NB: Also You can Read Class 12 chemistry practice paper 2023
SECTION B
17. (a) Define melal depression constant. How is it related to enthalpy of fusion ?
OR
(b) What type of deviation is shown by ethanol and acetone mixture? Give reason. What type of azeotropic mixture is formed by that deviation?
18. (a) In a reaction, if the concentration of reactant ‘X’ is tripled, the rate of reaction becomes twenty-seven times. What is the order of the reaction?
(b) State a condition under which a bimolecular reaction is kinetically a first-order reaction. Give an example of such a reaction.
(b) What type of deviation is shown by ethanol and acetone mixture? Give reason. What type of azeotropic mixture is formed by that deviation?
18. (a) In a reaction, if the concentration of reactant ‘X’ is tripled, the rate of reaction becomes twenty-seven times. What is the order of the reaction?
(b) State a condition under which a bimolecular reaction is kinetically a first-order reaction. Give an example of such a reaction.
19. Complete the following ionic equations:
(a) 2MnO4^- + 5SO3^-2 + 6H^+ –>
(b) Cr2O7^2- + 14H^+ + 6Fe^2+ –>
20. (a) Which halogen compound in the following pair will react faster in SN2 reactions and why?
CH3-CH2-I or CH3-CH2-Br
(b) Why is chloroform stored in closed dark coloured bottles?
21. Give the reaction of glucose with the following:
(a) HCN
(b) Conc. HNO3
All the Questions are from NCERT Chemistry Book, so better prepare ncert book and make notes.
SECTION C
22. A solution is prepared by dissolving 5 g of a non-volatile solute in 200 g of water. It has a vapour pressure of 31.84 mm Hg at 300 K. Calculate the molar mass of the solute.
(Vapour pressure of pure water at 300K = 32 mm Hg)
23. The conductivity of 0.2 M solution of KCI is 2.48×10^-2 S cm-¹. Calculate its molar conductivity and degree of dissociation.
Given: Equivalent Conductance of K+ = 73.5 S cm^2/mol
Equivalent Conductance of Cl- = 76.5 S cm^2/mol
For more questions you can click the pdf file here CBSE Class 12 Chemistry Question paper 2024 pdf set-1
Find more from Syllabuswise.com
