Here you will find the NCERT Chemistry Class 10 Chapter 1 notes name of the chapter :
Chemical reactions are the processes in which new substances with new properties are formed. Only a rearrangement of atoms takes place in a chemical reaction. The substances which take part in a chemical reaction are called reactants. … In a chemical reaction, reactants are transformed into products.
Visually Observable Characteristics
1. Change in color.- One of the most easily observable changes, which may take place during a chemical reaction, is a color change.
Of course, if two different colored liquids combine, they will form a new color. This is not an indicator of a chemical reaction. If a new color emerges after a few seconds or minutes, however, then a chemical reaction may have taken place.
Example- Rust forms on your bike when the iron in the metal frame reacts with the oxygen and water in the air. It forms a new compound called iron oxide. This change in color, from blue to reddish brown, indicates that a chemical change has taken place.
2. Temperature Change
When energy is either absorbed or released, it indicates a chemical change.
Ex- Fireworks are an example of a chemical change that produces a temperature change and emits light.
3. Gas Production
The production of gas is a clear sign that a chemical change has occurred. A quite delicious example of that is baking a cake. When you bite into a moist cake and see the tiny holes in the cake, this is indicative of the rising agent (either baking soda or baking powder) reacting with the acidic components of the cake to create carbon dioxide. This gas is what helps a cake rise in the oven.
4. Change of state-In some reactions, a change of a state is observed. The reaction starts with solid or liquid reactants and ends up with gaseous products and vice versa.
Example: Ammonia gas reacts with hydrogen chloride gas to form ammonium chloride which is in the solid state.
NH3(g) + HCl(g) –> NH4Cl(s)
A balanced chemical reaction is one in which the no. of atoms present in the reactant side are equally present on the product side. Because of the Law of Conservation of Mass, chemical equations must be balanced.
STEPS for Balancing Chemical Equations -There are four basic steps
1. Write the correct formula for the reactants and the products
2. Find the number of atoms for each element on the left side and Compare those against the number of atoms of the same element on the right side.
3. Determine where to place coefficients in front of formulas – The left side must have the same number of atoms as the right side for each element to balance the equation
4. Check your answer to see if: •The numbers of atoms on both sides of the equation are now balanced •
Chemical Reactions and Equation. The part of A combination reaction is a chemical reaction in two or more substances combine together to produce a new compound.
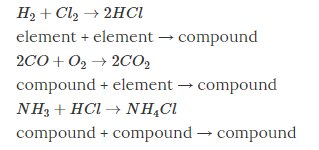
A single substance as a product is the key characteristic of the combination reaction.
For ex- 2 H2(g) + O2(g) → 2 H2O(ℓ)
Most combination reactions are exothermic in nature. Combination reactions involve the formation of new bonds and this process releases a large amount of energy in the form of heat.
Ex-i)Burning of Magnesium ribbon
2Mg+O2→2MgO+Heat
ii)Formation of slaked lime
CaO + H2O→Ca(OH)2+Heat
Respiration is considered an exothermic reaction:
- During respiration, glucose combines with oxygen in the cells of our body to form carbon dioxide and water along with the production of energy.
Glucose + oxygen → carbon dioxide + water + energy
It is an exothermic reaction because energy is produced during this process
Decomposition reaction
A single reactant decomposes on the application of heat or light or electricity to give two or more products.
Decomposition reactions can be classified into three types:
- Thermal decomposition reaction.
- Electrolytic decomposition reaction.
- Photo decomposition reaction.
Thermal decomposition reaction A thermal decomposition reaction can be defined as a decomposition reaction which is activated by thermal energy. The reaction is generally endothermic as energy is required to break chemical bond in order to separate the constituent elements.
a. Decomposition reactions which require heat – thermolytic decomposition or thermolysis.
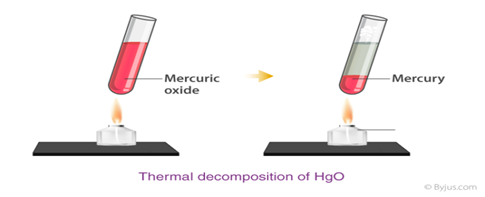
When heated Calcium Carbonate, decomposes into Calcium Oxide and Carbon Dioxide. This process is employed in the manufacturing of quick lime which is an important component in industries.
- CaCO3 → CaO + CO2
CBSE Class 10 Chemical Reactions and Equations notes
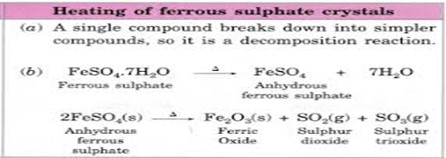

On heating, ferrous sulphate crystals lose water and anhydrous ferrous sulphate (FeSO4) is formed. So their colour changes from light green to white. On further heating, anhydrous ferrous sulphate decomposes to form ferric oxide (Fe2O3), sulphur dioxide (SO2) and sulphur trioxide (SO3).
When lead nitrate is heated, it decomposes into lead oxide, nitrogen dioxide and oxygen.
Lead nitrate crystals on strong heating decompose to form lead monoxide, nitrogen dioxide gas and oxygen gas. … The heating of lead nitrate does produce toxic fumes of lead and nitrogen dioxide.
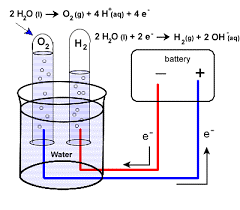
Electrolytic decomposition reaction
- An electrolytic decomposition reaction is a type of decomposition reaction in which the activation energy for decomposition.
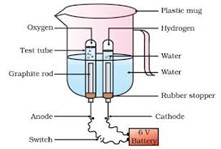
Example of electrolytic decomposition reaction
2 H2O(l) → 2 H2(g) + O2(g)
The number of hydrogen molecules produced is thus twice the number of oxygen molecules.
Photolysis or Photo Decomposition:
Reactions in which a compound decomposes because of sunlight are known as photolysis or photo decomposition.
Example:
When silver chloride is put in sunlight, it decomposes into silver metal and chlorine gas.

Similarly, when silver bromide is put under sunlight, it decomposes into silver metal and bromine gas.

Photographic paper has a coat of silver chloride, which turns into grey when exposed to sunlight. It happens because silver chloride is colourless while silver is a grey metal.
Also Solve the Class 10 Chapter-2 Acid Base & Salt Chapter MCQs
Science class 10 chapter 1 notes
Displacement Reactions may be of two types
Single Displacement
Double Displacement
- Single Displacement Reaction-
A single-displacement reaction is a chemical reaction in which one (or more) element(s) replaces another element(s) in a compound. It can be represented generically as:
A + B-C → A-C + B
This will most often occur if A is more reactive than B, thus giving a more stable product. A and B must be either:
- Different metals (hydrogen’s behavior as a cation renders it as a metal here), in which case C represents an anion;
The reactivity of Metals can be known from the Reactivity Series,
where Metals are arranged in the decreasing order of their reactivities.
(Hydrogen’s behaviour as a cation renders it as a metal here). –
K, Na, Ca, Mg, Al, Zn, Fe, Ni, Sn, Pb, [H], Cu, Hg, Ag, Au, and Pt.}
- Halogens, in which case C represents a cation.
Both metals and non-metals take part in displacement reactions.
- When Element C is a metal :
Example :
- Reaction of iron nails with copper sulfate solution-
Fe + Cu(SO4)2 -> Fe(SO4)2 + Cu(s) [Fe>Cu]
Blue sol. Pale green sol.
Observations-
- The blue colour of the solution changes to pale green due to the formation of FeSO4.
- Reddish brown Cu gets deposited on the iron nails.


- Reaction of copper coin with silver nitrate solution-
Cu + 2AgNO3 –> 2Ag(s) + Cu(NO3)2 [Cu>Ag]
colourless sol blue sol.
Observations-
- Colour less solution turns blue due to the formation of Cu(NO3)2.
- Silver gets deposited on the Cu coin.
- Reaction of calcium with water solution-
Ca + 2H2O -> Ca(OH)2 + H2(g) [Ca>H]
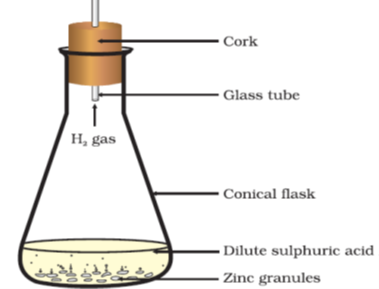
- Reaction of zinc granules with hydrochloric acid solution-
Zn + 2HCl -> ZnCl2 + H2(g) [Zn>H]
Observation for Ex. 3 & 4-
- Fizzing occurs due to the liberation of Hydrogen, which burns with a pop sound when a lighted match stick is brought near the delivery tube.
- The conical flask turns warm as it’s an exothermic reaction.
If the reactant in elemental form is not a more reactive metal, then no
reaction will occur.
Some examples of this would be the reverse reactions to these:
- Ag + Cu(NO3)2 → No reaction [Ag<Cu]
- Au + HCl → No reaction [Au<H]
Chemistry class 10 chapter 1 notes pdf download
- When Element C is a Halogen :
One halogen/anion replaces another. A Halogen/anion is a negatively charged ion or a non-metal.
Some of the few examples that involve halogens are shown here:
- Cl2 + 2NaBr -> 2NaCl + Br2 [Cl>Br]
- Br2 + 2KI -> 2KBr + I2 [Br>I]
Again, the less reactive halogen cannot replace the more reactive halogen:
Br2 + 2KCl -> No reaction. [Br<Cl]
- Double Displacement Reaction-
A reaction in which two compounds in their aqueous solution react to form two new products by interchanging their radicals or ions is called a double decomposition reaction or double displacement reaction
A-B + C-D → A-D + C-B
The bond between the reacting species can be either ionic or covalent.
Classically, these reactions may be of two types- Precipitation Reaction & Neutralization Reaction
- Precipitation Reaction –
A precipitation reaction is that in which one of the products of a Double displacement reaction is an insoluble solid which separates out as precipitate.
AX(aq) + BY(aq) → AY(aq) + BX(s).
Examples-
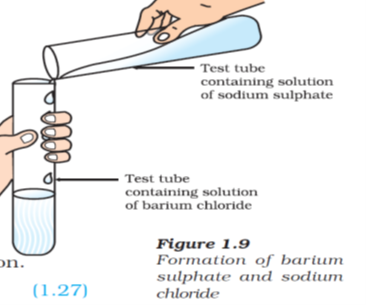
- Reaction of BaCl2 with Sodium Sulphate-
BaCl2(aq) + Na2SO4(aq) —–> BaSO4 (s)+ 2NaCl(aq)
(white ppt)
- Reaction of Lead Nitrate with Potassium Iodide-
Pb(NO3)2 + 2KI –> PbI2 + 2KNO3
( Yellow ppt)
- Neutralization Reaction –
Neutralisation reaction is that in which an acid reacts with a base to form salt & water.
HX(aq) + BOH(aq) → BX(aq) + H2O(l).
Acid + Base Salt Water
Examples-
- HCl + NaOH à NaCl + H2O
- H2SO4 + Mg(OH)2 à MgSO4 + 2 H2O
SOLUBILITY CHART
| SOLUBLE-SALTS | INSOLUBLE –SALTS (in cold water) |
| All – Na+, K+, NH4+ SALTS | 1 |
| 2.ALL- Nitrates, Nitrites | 2.ALL- NITRATES,NITRITES |
| 3.ALL- Bicarbonates | 3. KHCO3 , NaHCO3-sparingly soluble |
| 4. ALL- Sulphates | 4. PbSO4, AgSO4, CaSO4, BaSO4 |
| 5. ALL- Chlorides | 5.PbCl2, AgCl, HgCl,(PbCl2 soluble in hot water) |
| (All Na+, K+, NH4+– SULPHITES, SULPHIDES, CARBONATES, OXIDES, HYDROXIDES and POHOSPHATES ARE SOLUBLE.) | 6.ALL-SULPHITES |
| 7.ALL- SULPHIDES | |
| 8.ALL-CARBONATES | |
| 9.ALL-OXIDES | |
| 10.ALL-HYDROXIDES | |
| 11.ALL-POHOSPHATES |
All Read the CBSE Class 12 Electrochemistry important questions.
.
