Here are some CBSE Class 12 Electrochemistry MCQs
- Which cell will measure standard electrode potential of copper electrode?
(i) Pt (s) | H2 (g,0.1 bar) | H+ (aq.,1 M) || Cu2+(aq.,1M) | Cu
(ii) Pt(s) | H2 (g, 1 bar) | H+ (aq.,1 M) || Cu2+ (aq.,2 M) | Cu
(iii) Pt(s)| H2 (g, 1 bar) | H+ (aq.,1 M) || Cu2+ (aq.,1 M) | Cu
(iv) Pt(s) | H2 (g, 1 bar) | H+ (aq.,0.1 M) || Cu2+ (aq.,1 M) | Cu
- Which of the following statement is correct?
(i) ECell and ΔrG of cell reaction both are extensive properties.
(ii) ECell and ΔrG of cell reaction both are intensive properties.
(iii) ECell is an intensive property while ΔrG of cell reaction is an extensive property.
(iv) ECell is an extensive property while ΔrG of cell reaction is an intensive property.
- The difference between the electrode potentials of two electrodes when no current is drawn
through the cell is called
(i) Cell potential
(ii) Cell emf
(iii) Potential difference
(iv) Cell voltage
- Which of the following statement is not correct about an inert electrode in a cell?
(i) It does not participate in the cell reaction.
(ii) It provides surface either for oxidation or for reduction reaction.
(iii) It provides surface for conduction of electrons.
(iv) It provides surface for redox reaction.
CBSE class 12 electrochemistry important questions
- An electrochemical cell can behave like an electrolytic cell when
(i) Ecell = 0
(ii) Ecell > Eext
(iii) Eext > Ecell
(iv) Ecell = Eext
- Which of the statements about solutions of electrolytes is not correct?
(i) Conductivity of solution depends upon size of ions.
(ii) Conductivity depends upon viscosiy of solution.
(iii) Conductivity does not depend upon solvation of ions present in solution.
(iv) Conductivity of solution increases with temperature.
Also Practice: CBSE Class 12 Chemical Kinetics MCQs
7.Using the data given below find out the strongest reducing agent.
(i) Cl–
(ii) Cr
(iii) Cr3+
(iv) Mn2+
8.Use the data given in Q.7 and find out which of the following is the strongest oxidising agent.
(i) Cl–
(ii) Mn2+
(iii) MnO–4
(iv) Cr3+
9.Using the data given in Q.7 find out in which option the order of reducing power is correct.
(i) Cr3+ < Cl– < Mn2+ < Cr
(ii) Mn2+ < Cl– < Cr3+ < Cr
(iii) Cr3+ < Cl– < Cr2O72- < MnO–4
(iv) Mn2+ < Cr3+ < Cl– < Cr
10.Use the data given in Q.7 and find out the most stable ion in its reduced form.
(i) Cl–
(ii) Cr3+
(iii) Cr
(iv) Mn2+
11.Use the data of Q.7 and find out the most stable oxidised species.
(i) Cr3+
(ii) MnO–4
(iii) Cr2O72-
(iv) Mn2+
11.The quantity of charge required to obtain one mole of aluminium from Al2O3 is
(i) 1F
(ii) 6F
(iii) 3F
(iv) 2F
12.The cell constant of a conductivity cell
(i) changes with change of electrolyte.
(ii) changes with change of concentration of electrolyte.
(iii) changes with temperature of electrolyte.
(iv) remains constant for a cell.
13.While charging the lead storage battery
(i) PbSO4 anode is reduced to Pb.
(ii) PbSO4 cathode is reduced to Pb.
(iii) PbSO4 cathode is oxidised to Pb.
(iv) PbSO4 anode is oxidised to PbO2.
15. During the electrolysis of aqueous NaCl, the cathodic reaction is (CBSE All India-2024)
(A) Oxidation of Cl- ion
(B) Reduction of Na+ ion
(C) Oxidation of H2O
(D) Reduction of H2O
Click Here To download the complete/ chapter wise e-book of NCERT Chemistry
16.One Faraday of electricity will liberate one gram mole of the metal from the solution of:
(i)BaCl2 (ii) CuSO4 (iii) AlCl3 (iv) NaCl
- The amount of electricity that can deposit 108g of silver from silver nitrate solution is:
(i)1 ampere (ii)1 coulomb (iii) 1 faraday (iv) 2 ampere
- On increasing dilution,the specific conductance:
(i)increases (ii) decreases (iii) remains constant (iv)none of these.
- The distance between two electrodes of a cell is 2.5cm and area of each electrode is 5cm2.The
cell constant is:
(i) 2 (ii) 12.5 (iii) 7.5 (iv) 0.5
- The cathodic reaction in electrolysis of dil.H2SO4 with platinum electrode is:
(i) oxidation (ii)reduction (iii)both oxidation and reduction (iv) neutralisation
- The charge required to deposit 9g of Al from Al3+solution is :
(i) 3216.3C (ii) 96500C (iii) 9650C (iv) 32163C
- The amount of electricity required to produce one mole of copper from copper sulphate solution
Is: (i) 1F (ii)2.33F (iii)2F (iv)1.33F
- As a lead storage battery is charged,then:
(i)lead dioxide dissolves (ii)H2SO4 is generated (iii)lead electrode becomes coated with lead
sulphate (iv)the concentration of H2SO4 decreases
- What is the amount of chlorine evolved when 2 amperes of current is passed for 30min in an
aqueous solution of NaCl?
(i)66g (ii)1.32g (iii)33g (iv)99g
25.Which is the strongest reducing agent ?
(i)Zn (ii)Cr (iii)H2 (iv)Fe2+
- How much silver will be obtained by that quantity of current which displaces 5.6 litre of H2?
(i)54g (ii)13.5g (iii)20g (iv)108g
27.Hydrogen gas will not reduce:
(i)heated Cu2O (ii)heated Fe2O3 (iii)heated Sn2O3 (iv)heated Al2O3
28.What is the number of coulombs required for the conversion of one mole of MnO4– to Mn2+?
(i)482500C (ii)289500C (iii)9650C (iv)96500C
29.When one faraday of electricity is passed, the mass deposited is equal to:
(i)one gram equivalent (ii)one gram mole (iii)electrochemical equivalent (iv)half gram equivalent
30.A solution of sodium sulphate in water is electrolysed using Pt electrodes. The products at
cathode and anode are respectively:
(i)H2,O2 (ii)O2,H2 (iii)O2,Na (iv)O2,SO2
31.Which one is the correct equation that represents the first law of electrolysis?
(i)mZ=ct (ii)m=cZt (iii)mc=Zt (iv)c=mZt
CBSE Class 12 Electrochemistry sample MCQs
Note : In the following questions two or more than two options may be correct.
32.The positive value of the standard electrode potential of Cu2+/Cu indicates that
(i) this redox couple is a stronger reducing agent than the H+/H2 couple.
(ii) this redox couple is a stronger oxidising agent than H+/H2.
(iii) Cu can displace H2 from acid.
(iv) Cu cannot displace H2 from acid.
(i) In dilute sulphuric acid solution, hydrogen will be reduced at cathode.
(ii) In concentrated sulphuric acid solution, water will be oxidised at anode.
(iii) In dilute sulphuric acid solution, water will be oxidised at anode.
(iv) In dilute sulphuric acid solution, SO42- ion will be oxidised to tetrathionate ion at anode.
34.E °Cell=1.1V For Daniel cell, Which of the following expressions are correct description of state of equilibrium in this cell?
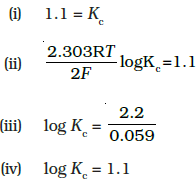
35.Conductivity of an electrolytic solution depends on
(i) nature of electrolyte.
(ii) concentration of electrolyte.
(iii) power of AC source.
(iv) distance between the electrodes.
36.
37.What will happen during the electrolysis of aqueous solution of CuSO4 by using platinum
electrodes?
(i) Copper will deposit at cathode.
(ii) Copper will deposit at anode.
(iii) Oxygen will be released at anode.
(iv) Copper will dissolve at anode.
38.What will happen during the electrolysis of aqueous solution of CuSO4 in the presence of Cu
electrodes?
(i) Copper will deposit at cathode.
(ii) Copper will dissolve at anode.
(iii) Oxygen will be released at anode.
(iv) Copper will deposit at anode.
39.Conductivity κ , is equal to
40.Molar conductivity of ionic solution depends on
(i) temperature.
(ii) distance between electrodes.
(iii) concentration of electrolytes in solution.
(iv) surface area of electrodes.
41.For the given cell, Mg|Mg2+|| Cu2+|Cu
(i) Mg is cathode
(ii) Cu is cathode
(iii) The cell reaction is Mg + Cu2+ → Mg2+ + Cu
(iv) Cu is the oxidizing agent

Comments are closed.